Efficient Catalysts for Green Synthesis of Adipic Acid from Biomass
Weiping Deng, Longfei Yan, Binju Wang, Qihui Zhang, Haiyan Song, Shanshan Wang, Qinghong Zhang, and Ye Wang
Angewandte Chemie International Edition
DOI: 10.1002/anie.202013843
Article Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202013843
研之成理推送:https://mp.weixin.qq.com/s/FEBvhxz-0rqDAwPkB4oX6w
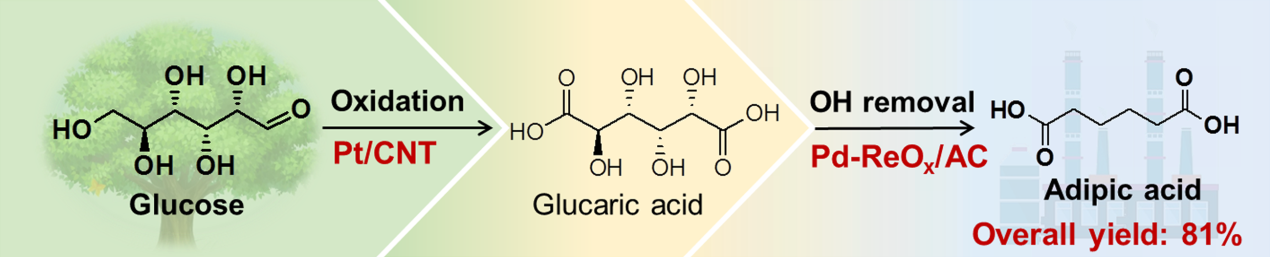
Green synthesis of adipic acid from renewable biomass is a very attractive but challenging goal of sustainable chemistry. In a recent article published in Angewandte Chemie International Edition, we report a combination of heterogeneous catalysts for the synthesis of adipic acid from glucose via glucaric acid with an overall yield of 81%.
Adipic acid is a key monomer for synthesis of important polymers such as nylon-66 and is one of the most important synthetic intermediates in the chemical industry. Currently, adipic acid is primarily produced by a multi-step process, including the hydrogenation of petroleum-derived benzene to cyclohexane, the subsequent oxidation of cyclohexane into a mixture of cyclohexanone and cyclohexanol (KA oil), and the further oxidation of KA oil to adipic acid by nitric acid. This process not only suffers from high energy-consumption but also has problems of emission of N2O and low efficiency due to the low single-pass yield (<10%) of KA oil in cyclohexane oxidation. The development of novel processes for green synthesis of adipic acid becomes urgent because of the increasing need for building green and sustainable chemical society.
On the other hand, the catalytic valorization of renewable biomass into high-value chemicals has attracted much attention in recent years. As the major component of lignocellulosic biomass, the largest renewable carbon resource on the earth, cellulose, which consists of glucose units connected by β-1,4-glycosidic linkage, is an ideal feedstock for sustainable production of organic oxygenated compounds. In particular, cellulose holds the potential for the synthesis of adipic acid because of the six-carbon skeleton of glucose monomer. In addition, sugars including glucose that can be derived from other biomass resources would also play important roles in the sustainable production of chemicals. A few studies have been devoted to the synthesis of adipic acid or adipate esters through catalytic transformations of biomass-derived C6 dicarboxylic acids such as 2,5-furandicarboxylic acid (FDCA) and mucic acid. However, these C6 dicarboxylic acids are not easily available, and multiple chemical-reaction or biological-fermentation processes are required. The synthesis of adipic acid from easily available C6 sugars, in particular glucose, with one or two steps is a fascinating route, but so far only very limited success has been achieved.
We have succeeded in develop new tandem catalytic systems for the synthesis of adipic acid from glucose via glucaric acid with an overall yield of 81%. Glucose is first oxidized into glucaric acid, which undergoes Deoxydehydration (DODH) reaction to form adipic acid after removal of four OH groups. Carbon nanotube-supported platinum nanoparticles (Pt/CNT) were found to be an efficient catalyst for the selective oxidation of glucose to glucaric acid, offering a glucaric acid yield of 82%. The catalytic behaviors of Pt/CNT were dependent on the size of Pt nanoparticles, kinetic parameters (such as temperature and reaction time) and the pH of the reaction solution. Gluconic acid and glucuronic acid were the reaction intermediates, and the oxidation of gluconic acid was a crucial step.
We discovered that a bifunctional catalyst composed of rhenium oxide and palladium on activated carbon (Pd−ReOx/AC) was very efficient for the removal of four OH groups in glucaric acid to adipic acid in the form of methyl adipate in methanol and the yield of adipic acid reached 99%. The highly dispersed ReOx species with rhenium in a high oxidation state (i.e., ReVI) functions for the DODH reaction, while Pd nanoparticles not only catalyze the hydrogenation of C=C bonds in the intermediates but also synergistically facilitate the DODH reaction probably by enhancing the dispersion of ReOx species and the reduction of ReOx species. Our DFT calculations suggest that the binuclear Re−O−Re site with one rhenium in five-coordination is responsible for the DODH reaction and the Pd nanoparticles promote both the dehydration of diols and the regeneration of the oxidized ReOx sites under H2, providing deep understanding of the synergistic effect between ReOx and Pd nanoparticles. The Pd−ReOx/AC catalyst could also be applied to the DODH reaction of several other biomass-derived platform molecules.
The present work not only presents powerful catalysts to accomplish the green synthesis of adipic acid from glucose but also offers an effective bifunctional-catalysis strategy to remove multiple hydroxyl groups for biomass valorization.