Boosting chemical and fuel production
by Cao-Thang Dinh, Queen’s University, Canada
Article Link: https://www.nature.com/articles/s41929-020-0467-4
Our recent work in Nature Catalysis (2020, 3, 478-487) was highlighted by Cao-Thang Dinh, from Queen’s University of Canada. He gave a very positive comment on our work. Such as, “Catalysts are the heart of CO2 electroreduction technology. Now, a catalyst has been developed that converts CO2 into C2+ products with very high selectivity, stability, and energy efficiency at industrially relevant current densities.” “Writing in Nature Catalysis, Wang, Cheng, Zhang, and co-workers now report a Fluorine-doped copper (F–Cu) catalyst that converts CO2 into C2+ with impressive selectivity and energy efficiency at a current density exceeding 1 A cm–2. The catalysts are also stable, opening the door to further development of practical CO2 electroreduction technology.” “Remarkably, this high current density was achieved with a C2+ Faradaic efficiency of 85% and with an applied potential as low as –0.67 V versus the reversible hydrogen electrode, indicating that F–Cu is among the most active and selective catalysts for C2+ production.” “Wang and co-workers found that the hydrogenation of *CO to *CHO occurs with a low kinetic barrier on the F–Cu surface. Thus, the hydrogenation of *CO to *CHO, followed by the subsequent coupling of *CHO, becomes the most favourable pathway for the formation of C2+ on the F–Cu catalyst.”
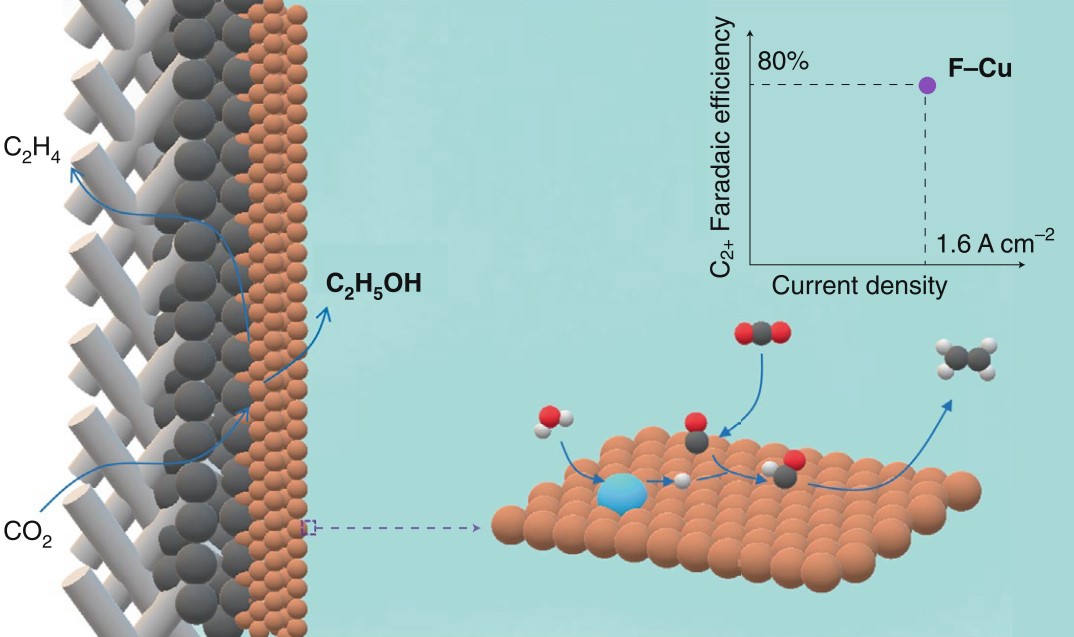
Fig. 1 | CO2 electroreduction on F–Cu catalysts loaded on a gas diffusion electrode. Structure of the gas diffusion electrode and proposed reaction mechanism on the surface of F–Cu catalysts.